Current news: Good news. On April 3, 2020, the EU Commission officially accepted the proposal to postpone the start of the MDR by 2021.
On April 17, 2020, the EU parliament approved the postponement.
Use the time you have saved! We also support you in the corona crisis by the implementation of MDR requirements so that your employees can fully focus on the production of urgently needed medical devices.
Our MDR Ticker (german) keeps you up to date. You can contact us also by email. We will be happy to send you the latest information about the MDR and other relevant themes as soon as we get it.
Our services
We will be pleased to support you with your:
- Literature research
- Technical Documentation
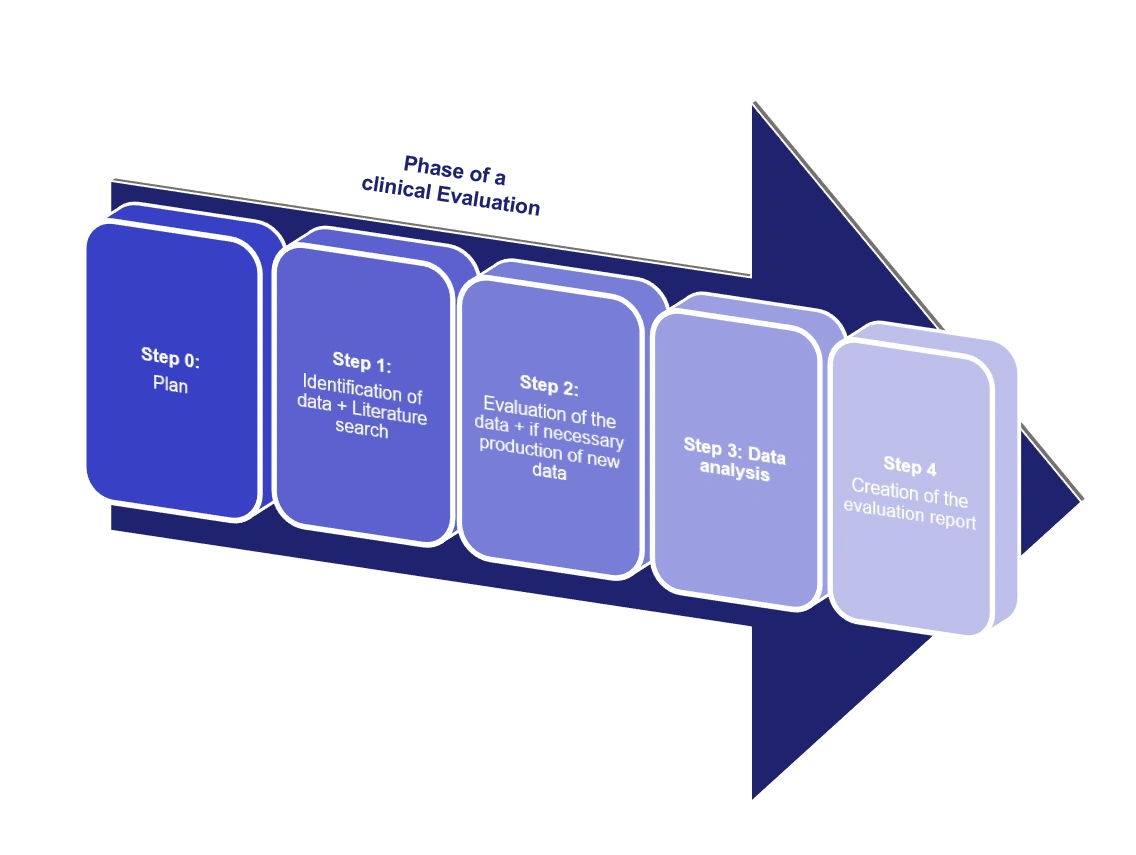
- Clinical evaluation
- Review your clinical evaluation
- Market surveillance after placing on the market (PMS)
- Post-market clinical follow up (PMCF studies)
- Regular update service for your clinical evaluation
Contact us and we will we will prepare you an offer tailored to your needs!
Your advantages at a glance
The key benefits offered to our clients are:
Commissioning us as an external service provider means saving you time, costs and resources, since you do not have to hire any extra-qualified personnel. Your employees can concentrate their full attention on their core business, the development and manufacture of innovative medical products, while we as a qualified service provider support you by or relieve you of the time-consuming, regulatory work.
Why do our clients delegate part of the regulatory tasks to us:
- Time saving (shorter processing time by external specialists)
- Use of the expertise of experienced professionals
- Saving resources (personnel, equipment)
- high planning reliability through goal-oriented project processing
- Cost savings (qualified personnel can work on the development of new products, regulatory work is carried out by external experts)

Contact
You can reach us by phone at +49 5602 9183940, by email: web@ancura.eu or use our contact form.
Dr. Christina Heller
CEO AnCura Medical Consulting GmbH
Literaturesearch
We search the appropriate literature sources for you.
Alexander Pax
CEO AnCura Medical Consulting GmbH
Clinical evaluation
We create the appropriate evaluation for your medical device.
Clinical trial
We support you in conducting your medical device clinical trial.
About us
Founded in Hessisch Lichtenau in 2019, AnCura Medical Consulting GmbH specializes primarily in the creation of clinical evaluations and PMCF studies. We also offer a wide range of clinical research (CR) services.
Due to the size of our company and the resulting lean organization, we can primarily work on projects with a small and medium budget and that in the highest scientific and methodological quality. But also large companies find the ideal contact in us, because we work intensively on every project, no matter how extensive it is. We support you in concentrating all your attention on the core business, the development of innovative medical devices.
In order to be able to complete all projects with the highest possible scientific standard, we work together closely with a network of specialists.
The graduated biologist Alexander Pax founded the company. Mr. Pax has 14 years of professional experience in conducting clinical evaluations and studies for medical devices of classes I to III. During this time, he has performed more than 250 clinical evaluations for a variety of medical devices. Among others for the following indications:
- General and plastic surgery
- Diabetology
- Implantology
- Intensive care medicine
- Cardiology
- Nephrology
- Neurology
- Oncology
- Orthopedics, orthopedic surgery
- Rheumatology
He is supported by the second managing director Dr. rer. nat. Christina Heller. She has almost 20 years of experience in the field of scientific publications, literature searches and data analysis (including biostatistics). She is also responsible for quality management (TÜV-certified) and the office organization (finance, resources). She supports Mr. Pax in the preparation of the clinical evaluations.
Our company works according to the current guidelines and recognized quality standards. Our company works according to the current guidelines and recognized quality standards. We attach great importance to the consistent implementation of the high standards of DIN EN ISO 9001 and to all questions of data protection.
You can contact us at any time. Personal and individual support tailored to your needs is important to us.
Medical device regulation (MDR)
We invite you to get a deeper insight into the exciting subject area of the new Medical Device Regulation (MDR). We look forward to supporting you.


