Topical: EU Commission wants to improve MDR
The time has come! From now on, the requirements of EU Regulation 2017/745 (MDR) apply.
In order to maintain the availability of medical devices on the EU market, the EU Commission plans to improvements to EU Regulation 2017/745 (MDR).
Our MDR Ticker (german) keeps you up to date. Or contact us by email. We will be happy to send you new information as soon as we have it.
Conducting clinical evaluations according to the MDR (2017/745) and MedDev 2.7/1 Rev.4
Clinical evaluation of medical devices – regulations
Clinical evaluation
With the help of the clinical evaluation, all manufacturers of medical devices (Class I to III) must demonstrate that the benefits, performance and safety of the devices are adequate. In other words, it must be ensured that the product does not compromise either the clinical condition or the safety of patients or third parties. Each medical device must therefore either still meet the essential requirements (ER) of the MDD or meet the essential safety and performance requirements (GSPR) of the MDR.
A clinical evaluation is composed of the following documents:
- clinical evaluation plan (CEP) including the clinical development plan (if necessary) and
- clinical evaluation report (CER).
How the clinical evaluation should be structured and what content it must contain is described in numerous regulations and standards. These include, among others, Annex X of the Medical Device Directive (MDD); Articles 10 and 61, as well as Annex XIV of EU Regulation 2017/745 (MDR), Paragraph 19 of the Medical Devices Act (MPG) and MEDDEV 2.7.1.
In addition to the CEP and CER, the documentation must contain a number of other documents. The type and scope of this documentation depends on the class of the medical device (see figure). The risk management file, for example, should also be included here, since risk management is an essential prerequisite for clinical evaluation and vice versa.
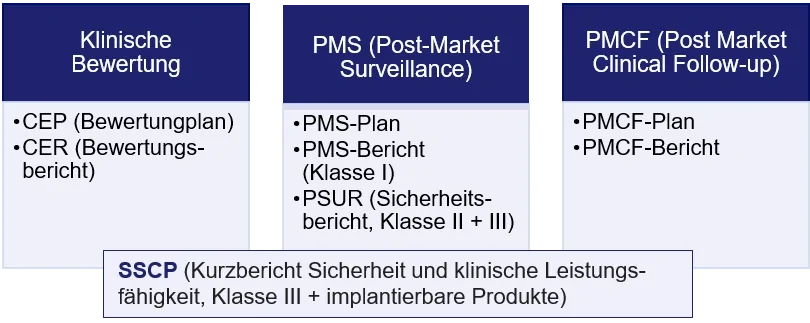
Clinical data
Clinical data form the basis of any clinical evaluation. These data are used to evaluate whether the medical device is safe and performs well, and also allow the manufacturer to assess whether the risks encountered in connection with use are commensurate with the expected benefits of the device. The data can come from numerous sources, e. g.:
- Results of clinical trials (by the manufacturer)
- Scientifically relevant literature on the product or the selected equivalent products (e.g., results of clinical trials or articles on other clinical experience with the product)
- Clinically relevant results from post-market surveillance (PMS) and post-market clinical follow up (PMCF).
- Reports of incidents with the product or equivalent products in the relevant databases (BfArM, FDA (MAUDE and TPLC) and other).
Notified bodies expect manufacturers to use multiple sources for clinical evaluation. However, the scope of the clinical evidence must be based on the characteristics of the product and its intended use. The scope for a standard Class I product from infection control, for example, may be less than for a Class III product. Manufacturers are therefore required to specify and justify the scope of the selected clinical data (clinical evidence). They must also demonstrate that the selected clinical data are sufficient to meet the essential safety and performance requirements. (Source: https://www.bundesgesundheits-ministerium.de/naki.html).
Is it possible to dispense with a clinical evaluation?
Neither the Medical Device Directive (MDD), nor the EU Regulation 2017/745 (MDR) allow the renouncing of a clinical evaluation. However, under certain conditions, clinical evaluation based on clinical data can be renounced. However, this usually only applies to absolutely non-critical products.
The MDR states in this regard in Article 61, paragraph 10:
Without prejudice to paragraph 4, where the demonstration of conformity with general safety and performance requirements based on clinical data is not deemed appropriate, adequate justification for any such exception shall be given based on the results of the manufacturer's risk management and on consideration of the specifics of the interaction between the device and the human body, the clinical performance intended and the claims of the manufacturer. In such a case, the manufacturer shall duly substantiate in the technical documentation referred to in Annex II why it considers a demonstration of conformity with general safety and performance requirements that is based on the results of non-clinical testing methods alone, including performance evaluation, bench testing and pre-clinical evaluation, to be adequate.
The process of clinical evaluation
According to MDR, Article 61, Section 3, a clinical evaluation shall follow defined and methodologically sound procedure based on the following:
- critical evaluation of the relevant scientific literature currently available relating to the safety, performance, design characteristics and intended purpose of the device, where the following conditions are satisfied:
- it is demonstrated that the device subject to clinical evaluation for the intended purpose is equivalent to the device to which the data relate, in accordance with Section 3 of Annex XIV, and
- the data adequately demonstrate compliance with the relevant general safety and performance requirements;
- a critical evaluation of the results of all available clinical investigations, taking duly into consideration whether the investigations were performed under Articles 62 to 80, any acts adopted pursuant to Article 81, and Annex XV; and
- a consideration of currently available alternative treatment options for that purpose, if any.
Since June 2016, the exact procedure has been described in detail in the clinical evaluation guideline published by the European Commission (MEDDEV 2.7/1 rev. 4). A new feature is that the clinical evaluation plan, which defines the objectives and structure of the clinical evaluation, must in future also include a clinical development plan.
The clinical development plan, for example, describes in detail the clinical planning envisaged by the manufacturer in order to be able to demonstrate the clinical safety, the lowest possible burden and the effective benefit of the medical device to be evaluated via its own clinical data. Clinical planning ranges from exploratory to pivotal studies to post-marketing clinical follow-up (PMS), specifying milestones and describing possible acceptance criteria.
In general, according to the MDR, a clinical evaluation and associated documentation must be updated throughout the lifecycle of the device based on clinical data. For this purpose, the manufacturer needs a plan for the Post Market clinical follow-up in accordance with Annex XIV Part B of the MDR and Article 84 (see PMCF studies).
Briefly described, a clinical evaluation proceeds as follows:
- 0) Planning:
- Determine the regulatory requirements to be supported by the clinical data.
- 1) Identification:
- Research of all relevant data from the scientific literature.
- Data from the manufacturer and data from studies.
- State of the art within the given purpose of the product - what is currently the standard product for the selected purpose - comparison with the new product.
- 2) Data assessment:
- Evaluation of the individual data.
- Assess whether the available data are sufficient to demonstrate product safety.
- If necessary, further generation of clinical data to clarify open points with regard to safety and guidance.
- 3) Analysis:
- Prepare a summary clinical evaluation of the product on safety and performance.
- 4) Report
- Final report on the evaluation.
Clinical evaluation - equivalence route
According to the MDR, the concept of similarity to other products can still be applied to products for which clinical data are already available, but only in a limited number of situations. The new rules are also stricter (MDR, Article 61, 3 MDR, and Annex XIV, paragraph 3). They state that:
A clinical evaluation may be based on clinical data relating to a device for which equivalence to the device in question can be demonstrated. The following technical, biological and clinical characteristics shall be taken into consideration for the demonstration of equivalence:
- Technical: the device is of similar design; is used under similar conditions of use; has similar specifications and properties including physicochemical properties such as intensity of energy, tensile strength, viscosity, surface characteristics, wavelength and software algorithms; uses similar deployment methods, where relevant; has similar principles of operation and critical performance requirement.
- Biological: the device uses the same materials or substances in contact with the same human tissues or body fluids for a similar kind and duration of contact and similar release characteristics of substances, including degradation products and leachables
- Clinical: the device is used for the same clinical condition or purpose, including similar severity and stage of disease, at the same site in the body, in a similar population, including as regards age, anatomy and physiology; has the same kind of user; has similar relevant critical performance in view of the expected clinical effect for a specific intended purpose.
The characteristics listed in the first paragraph shall be similar to the extent that there would be no clinically significant difference in the safety and clinical performance of the device. Considerations of equivalence shall be based on proper scientific justification. It shall be clearly demonstrated that manufacturers have sufficient levels of access to the data relating to devices with which they are claiming equivalence in order to justify their claims of equivalence.
MDCG 2020-5 "Clinical Evaluation - Equivalence" can be used as a guideline by manufacturers and notified bodies to perform equivalence in accordance with the standard. In general, the requirements listed in the MDR (biological, technical, clinical) should be read very carefully, as the requirements can only be met in part if the same aspects are fulfilled or even if they are only similar.
In cases where equivalence cannot be demonstrated under the MDR, the data from similar devices may be useful for a variety of other purposes, for example (MDCG 2020-5):
- Ensuring that the risk management system is comprehensive by identifying relevant hazards and clinical risks.
- Understanding the state of the art, the natural course of disease and alternative available treatment options.
- Helping to define the scope of the clinical evaluation, by identifying any design features in similar devices that pose special performance or safety concerns.
- Provide input for clinical investigation design or post-market clinical follow-up design, and the post-market surveillance system.
- Identification of relevant and specified clinical outcome parameters for the intended clinical benefits, based on the published clinical data pertaining to the similar device(s).
- To define minimum requirements for a quantified clinical benefit that is considered clinically relevant, and/or to identify acceptable occurrence rates of risks and adverse events.
Please note that the MDCG guidance documents do not have a binding character for authorities or notified bodies. However, current practice shows that the suggestions of these documents are followed in the vast majority of cases.
In summary
As the MDR severely restricts equivalence assessment, this means that you, as a manufacturer, will need to generate and maintain more of your own clinical data for your products in order to be able to show clinical evidence with the essential safety and performance requirements as part of the clinical evaluation. You will need to account for this accordingly in the clinical development plan.
In addition, due to the limitations of equivalence consideration and the stringent requirements for the quality of clinical data, clinical trials for the initial marketing of a medical device and PMCF studies for recertification will be increasingly required. For Class III devices and implantable devices, these are even mandatory to perform, with a few exceptions (see below).
Therefore, as a manufacturer, you should critically review your own clinical data for the devices in this regard. If you identify gaps or find that your products are classified differently than before, or if you have to clinically evaluate your products for the first time, you should use the time until the end of May 2021 to actively generate clinical data on your own products via clinical trials or PMCF studies and to perform clinical evaluations.
The publications of the EU Medical Device Coordination Group (MDCG) provide some relief for manufacturers of legacy medical devices. In particular, MDCG 2020-6 and MDCG 2021-25 specify the applicable sources for clinical data for such "legacy devices". The requirements of the MDR for the PMS and, if necessary, for the PMCF must nevertheless be met in full.
Clinical evaluation of legacy devices
For existing devices, the so-called legacy devices, the MDCG has published the document MDCG 2020-6: "Regulation (EU) 2017/745: Clinical evidence needed for medical devices previously CE marked under Directives 93/42/EEC or 90/385/EEC". It is intended to serve as a guide for clinical data sufficient to provide evidence of compliance with the relevant GSPRs under Article 61(1) of the MDR for legacy devices.
Again, it should be noted that these guides are not binding on authorities or notified bodies.
According to MDCG 2021-25, legacy devices should be understood as devices, which, in accordance with Article 120(3) of the MDR, are placed on the market after the MDR’s date of application (DoA) and until 26 May 2024 if certain conditions are fulfilled. Those devices can be:
- Devices which are class I devices under Directive 93/42/EEC (MDD), for which an EC declaration of conformity was drawn up prior to 26 May 2021 and for which the conformity assessment procedure under the MDR requires the involvement of a notified body;
- Devices covered by a valid EC certificate issued in accordance with Directive 90/385/EEC (AIMDD) or the MDD prior to 26 May 2021.
‘Old’ devices are those devices that were placed on the market before 26 May 2021 in accordance with the AIMDD or the MDD or in accordance with the applicable rules before the Directives had entered into force (MDCG 2021-25).
The terminology “Well-established technology” is used in Article 52(5) and Article 61(8) of the MDR, but is not defined in these articles. The term is not restricted to the devices listed in Article 61(6b); Article 61(8) explicitly states that this includes devices similar to the exempted devices listed in Article 61(6b), which might be added to that list in future. The common features of the devices which are wellestablished technologies are that they all have:
- relatively simple, common and stable designs with little evolution; o their generic device group has well-known safety and has not been associated with safety issues in the past;
- well-known clinical performance characteristics and their generic device group are standard of care devices where there is little evolution in indications and the state of the art;
- a long history on the market. Daher können alle Produkte, die alle diese Kriterien erfüllen, als "etablierte Technologien" gelten (MDCG 2020-6).
When assessing the conformity of legacy devices under the MDR, it is important to verify whether PMCF studies considered necessary under the MDD/AIMDD (and where applicable, during the transition period, under the MDR), have been appropriately conducted, and results are taken fully into account for in the clinical evaluation for the conformity assessment under MDR (MDCG 2020-6).
Manufacturers are required to document a clinical evaluation plan to meet the requirements of MDR Annex XIV Section 1a.
A modified Clinical Evaluation Plan for legacy devices should include at least:
- An identification of the GSPR that require support from relevant clinical data.
- A specification of the intended purpose of the device.
- A clear specification of intended target groups with clear indications and contraindications.
- A detailed description of intended clinical benefits to patients with relevant and specified clinical outcome parameters.
- A strategy to identify, analyse and assess alternative treatments.
- A specification of methods to be used for examination of qualitative and quantitative aspects of clinical safety with clear reference to the determination of residual risks and side-effects.
- An indicative list and specification of parameters to be used to determine, based on the state of the art in medicine, the acceptability of the benefit-risk ratio for the various indications and for the intended purpose or purposes of the device.
- An indication how benefit-risk issues relating to specific components such as use of pharmaceutical, non- viable animal or human tissues, are to be addressed.
- A strategy and methodology to identify, analyse and appraise all relevant available clinical data in light of the changed definition for clinical data.
- Evidence for equivalence, if clinical data from an equivalent device is included in the clinical evaluation.
- A definition of the required level of clinical evidence, which shall be appropriate in view of the characteristics of the device and its intended purpose.
- A strategy and methodology to systematically collect, summarise and assess post market surveillance data to demonstrate continuing safety and performance, and to what extent complaints with regards to safety and performance have been observed with the legacy devices.
For the purpose of legacy devices, pre-market sources of clinical data may include (MDCG 2020-6):
- Clinical investigation reports of the device concerned.
- Clinical investigation reports or other studies reported in scientific literature, of a device for which equivalence to the device in question can be demonstrated in accordance with the MDR.
- Reports published in peer reviewed scientific literature on other clinical experience of either the device in question or a device for which equivalence to the device in question can be demonstrated.
- Other pre-market data, e.g. case reports on experience with the use of the device in question, such as compassionate or humanitarian exceptional use reports. Note that this kind of pre-market data may be more prone to bias, compared to those listed above.
It should be noted that MDR Article 2(48) provides a narrower definition of what constitutes clinical data sources as compared to the Directives which allow unpublished reports on other clinical experience to contribute to the clinical evaluation. Such data sources may provide informative context for the clinical evaluation of legacy devices.
Post-market sources of clinical data refer to data collected following the initial CE marking under the Directives (or prior to introduction of a new indication or design variant). This may include (MDCG 2020-6):
- PMS clinical data, complaint and incident reports.
- PMCF studies, including post-market clinical investigations.
- Independent clinical studies conducted using the device.
- Device registries.
- Data retrieved from the literature.
For well-established technologies the clinical evaluation can be based on data coming from similar devices, under the conditions detailed in paragraph 6.5 (e). With respect to legacy devices, when clinical data from equivalent devices is used, equivalence must be demonstrated according to the requirements of the MDR.
MDCG 2020-6 also proposes a hierarchy of clinical evidence to confirm compliance with the relevant GSPRs under the MDR, see Appendix II of the guidance document.
Class III and Implantable Devices - Evaluation and Clinical Trials
According to MDR Artikel 61, 4 in the case of implantable devices and class III devices, clinical investigations shall be performed, except if:
- the device has been designed by modifications of a device already marketed by the same manufacturer,
- the modified device has been demonstrated by the manufacturer to be equivalent to the marketed device, in accordance with Section 3 of Annex XIV and this demonstration has been endorsed by the notified body, and
- the clinical evaluation of the marketed device is sufficient to demonstrate conformity of the modified device with the relevant safety and performance requirements.
In this case, the notified body shall check that the post-market clinical follow-up plan is appropriate and includes post-market studies to demonstrate safety and performance of the device (MDR, Article 61, paragraph 4).
Article 61, paragraphs 5 and 6 state, that a manufacturer of a device demonstrated to be equivalent to an already marketed device not manufactured by him, may also rely on paragraph 4 in order not to perform a clinical investigation provided that the following conditions are fulfilled in addition to what is required in that paragraph:
- the two manufacturers have a contract in place that explicitly allows the manufacturer of the second device full access to the technical documentation on an ongoing basis, and
- the original clinical evaluation has been performed in compliance with the requirements of this Regulation
and the manufacturer of the second device provides clear evidence thereof to the notified body.
It is further valid that the requirement to perform clinical investigations pursuant to paragraph 4 shall not apply to implantable devices and class III devices,
a) which have been lawfully placed on the market or put into service in accordance with Directive 90/385/EEC or Directive 93/42/EEC and for which the clinical evaluation:
- is based on sufficient clinical data, and
- is in compliance with the relevant product-specific CS for the clinical evaluation of that kind of device, where such a CS is available; or
b) that are sutures, staples, dental fillings, dental braces, tooth crowns, screws, wedges, plates, wires, pins, clips or connectors for which the clinical evaluation is based on sufficient clinical data and is in compliance with the relevant product-specific CS, where such a CS is available.
In general, it is now only possible to dispense with a clinical evaluation under very specific conditions. Article 61, paragraph 10 of the MDR states in this regard:
Without prejudice to paragraph 4, where the demonstration of conformity with general safety and performance requirements based on clinical data is not deemed appropriate, adequate justification for any such exception shall be given based on the results of the manufacturer's risk management and on consideration of the specifics of the interaction between the device and the human body, the clinical performance intended and the claims of the manufacturer. In such a case, the manufacturer shall duly substantiate in the technical documentation referred to in Annex II why it considers a demonstration of conformity with general safety and performance requirements that is based on the results of non-clinical testing methods alone, including performance evaluation, bench testing and pre-clinical evaluation, to be adequate.
In principle, you as a manufacturer may now only waive a clinical evaluation for completely uncritical medical devices.
Sources:
- NAKI - National Working Group of the Federal Ministry of Health (Link)
- Full text of the consolidated version of the MDR (not official)
- Full text of the basic version MDR
- MDCG 2020-5 Clinical Evaluation - Equivalence A guide for manufacturers and notified bodies
- MDCG 2020-6 Regulation (EU) 2017/745: Clinical evidence needed for medical devices previously CE marked under Directives 93/42/EEC or 90/385/EEC A guide for manufacturers and notified bodies
- MDCG 2021-25 Regulation (EU) 2017/745 - application of MDR requirements to ‘legacy devices’ and to devices placed on the market prior to 26 May 2021 in accordance with Directives 90/385/EEC or 93/42/EEC
Clinical evaluation (CER and CEP)
Regulatory affairs for clinical evaluation
Clinical evaluation is a systematic and ongoing process that plays a role both in the product development phase and after the product has entered the market, right up to the end of the product life cycle.
In the context of clinical evaluation, we can therefore support you with the following services, so that your product receives approval quickly and your documentation is always up to date, even after market entry:
- Checking whether a clinical trial is necessary before approval and, if so, to what extent it should be carried out.
- Creation of the clinical evaluation plan incl. clinical development plan for your strategic planning.
- Reviewing, creating or revising/updating your clinical evaluation.
- Regularly reviewing the state of the art (state of the art) so that you can always respond to potential advancements and new developments.
- Conducting literature search using a comprehensible search strategy (such as PICO search), including summary and evaluation. Assessment of clinical data using evaluation schemes, such as JADAD, MINORS, etc.
- Update service for your clinical evaluation:
- Review at regular intervals for new literature at regular intervals.
- State of the art (SotA) reviews so you can respond quickly to new and evolving developments.
- Review of incident databases (BfArM, FDA (MAUDE and TPLC) and others).
- Advice on necessary updates.
- With our help you can:
- Avoid unnecessary work.
- Regularly adapt your risk analysis to new risks as they arise.
- Save time and money by bundling the time-consuming research work in one place.
With our help you can:
- Avoid unnecessary work
- Regularly adapt your risk analysis to new risks as they arise
- Save time and money by having the time-consuming research work bundled and evaluated in one place!
If we have aroused your interest, please contact us. Call us, write to us or fill out our contact form. We look forward to hearing from you.
- We work in compliance with MDR, MedDev 2.7/1 Rev. 4 and the MDD.
- We prepare your documents quickly and reliably in German or English using either in your or AnCura's own standard-compliant forms.
- We meet the general requirements for authors of clinical evaluations.
- We cooperate with a network of physicians / clinicians / statisticians.
- Together we find solutions, your satisfaction is our ultimate goal.
Regulatory affairs for clinical evaluation
Our services - Clinical evaluation (CER and CEP)
We support you as a manufacturer in every phase of the clinical evaluation of your medical device from setting up the clinical evaluation plan, to performing II a, IIb, b, III on.
This includes the following services:
Creating the clinical evaluation plan (CEP), planning the clinical evaluation.
We help you or create the clinical evaluation plan (CEP) required by the MDR. This must describe the exact objectives and structure of the clinical evaluation.Creation of the clinical development plan
The CEP must also include a clinical development plan. This describes how you, as the manufacturer, intend to demonstrate the clinical safety, lowest possible burden and effective benefit of your medical device based on your own clinical data. It includes the planned milestones and criteria for potential acceptance. Possible stages range from exploratory studies and pilot studies to post-marketing clinical surveillance (PMS). We support you by drawing up the plans (CEP, PMS, PMCF) in close consultation with you, developing milestones and planning possible studies for you.Literature research
According to the MDR, a clinical evaluation must follow a well-defined and methodologically sound procedure based on: a) a critical evaluation of the relevant currently available scientific literature on the safety, performance, design characteristics and intended purpose of the product. Here, too, we are happy to provide support, as the search must be conducted in the relevant databases, such as Embase, and takes a great deal of time. This includes creating the search plan, conducting the systematic search in the relevant databases, summarizing the data and making a recommendation as to whether this is sufficient for a clinical evaluation of your product or whether you should still generate data yourself.Conducting the clinical evaluation for your medical device
This includes all steps of a clinical evaluation from planning (search strategy, etc.), identifying and searching the relevant clinical data, evaluating the existing data (data from the manufacturer and from the literature), as well as a recommendation to you to generate new data if necessary, to analyzing the data and writing the final report. It is important for us to support you as a manufacturer on an individual basis. Since the requirements for the authors of a clinical evaluation have been tightened with the MDR and especially many small and medium-sized companies do not have the appropriate staff, you can entrust us with the planning and writing of your clinical evaluation. This leads to time, cost and resource savings for you as a manufacturer and you can fully concentrate on your strengths, the development of innovative medical devices.Updating your clinical evaluation
Updating the clinical evaluation is required by the MDR. In general, according to the MDR, a clinical evaluation and the associated documentation must be updated throughout the life cycle of the product based on the clinical data. Upon request, we will conduct a literature search for you at regular intervals or as needed following, for example, incidents, and bring new clinical data to your attention. If required, we can then also update the clinical evaluation or write a report why an update of the evaluation is not necessary.Review your clinical evaluation
If you, as a very small or small company, lack the specialist staff required by the MDR, but would still like to prepare your clinical evaluation yourself, we can subsequently check it for you to ensure that all the requirements of the MDR and MedDev 2.7/1 Rev. 4 have been met. There are a variety of possibilities here, contact us and we will find a solution.
For this purpose, we use our own forms created according to MedDev 2.7/1 Rev. 4, which we are also happy to provide to you. These were created according to the guidelines of DIN EN ISO 9001, which means that we regularly check and update them.
Generally, we offer our services in German as well as in English.
Contact us and we will be happy to create a customized offer for you.
FAQ
What is a clinical evaluation of medical devices?
Clinical evaluation is a methodologically sound, ongoing process for collecting, assessing and analyzing clinical data on a medical device. The purpose is to demonstrate that the benefits, performance and safety of the medical devices are adequate.
What documents does a Clinical Evaluation consist of?
A clinical evaluation consists of, among other things, the clinical evaluation plan including the clinical development plan (CEP) and the clinical evaluation report (CER). There should also be a literature search plan and report either as a separate document or as part of the CEP and CER.
Who is allowed to write / prepare a clinical evaluation?
The clinical evaluation should be conducted by an appropriately qualified person or team. The person or team should have, among other things, experience in research methodology and regulatory requirements, have, for example, knowledge of product technology and expertise in the relevant medical field, and have appropriate training (studies, work experience) (See MDR, Article 15 or MEDDEV 2.71 / rev.4, Section 6.4)

